
In the world of chemistry, structure can be the difference between life and death – quite literally. There are many compounds where slightly altering the structure or choosing a different enantiomer can make the compound go from a helpful medicine to a dangerous drug. That is why chemists are always very aware of the exact structure and certain enantiomers and their specific biological effects, because this information is very important in terms of determining the biological effects of compounds, good and bad!
First off, let’s begin with a short chemistry lecture. Chemical structure determines the molecular geometry of a compound by portraying the spatial arrangement of atoms and chemical bonds in the molecule. This provides chemists with an important visual representation of a chemical formula. Enantiomers are chiral molecules that are mirror images. In other words, enantiomers (pictured below) are the same compound with oppositely arranged chemical structures.
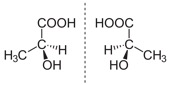
Now that we have the necessary background knowledge, we can discuss why enantiomers and structures are so important in the world of chemistry. Let’s start this discussion with a guessing game. Below are pictures of two different compounds with slightly different structures. As you can see, the only difference in structure is the two functional groups on the top and bottom left side of each. Both compounds are used as painkillers, however one is prescribed by professional doctors and the other is an illegal schedule 1 drug, and commonly abused for recreational purposes. Can you tell which is which?
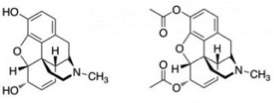
The compound on the left is called morphine, and the compound on the right is called diamorphine or diacetylmorphine, more commonly known as heroin. Because these two compounds have basically the same structure, they work in very similar ways; these compounds both act directly on the central nervous system to prevent pain signals from reaching the brain. However, the different functional groups on heroin make it considered more dangerous and produce euphoric effects as well.
Similarly, different enantiomers can produce very different biological effects of the same compound. An example of this is thalidomide. Thalidomide was used as an anti-morning sickness drug for pregnant women in the 1950s. It wasn’t until years later that thalidomide use was linked to serious birth defects and recalled. Scientists did not know why the drug caused birth defects while also producing positive anti-nausea effects as well, until they discovered that the two enantiomers had different biological effects on the body.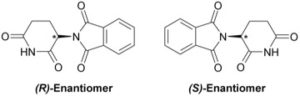
The two enantiomers of thalidomide, R and S, are mirror images of each other; the enantiomers are different chiral structures of the same compound, differing at the stereocenter (denoted by the asterisk). This case is different than the case of morphine versus heroin in the sense that these are the same compound rather than two similar but slightly different compounds; the enantiomers of thalidomide have the same chemical formula but are simply arranged differently. Because of the different spatial orientations, each enantiomer reacts differently with the body. This results in highly different side effects, some positive and some negative. Although thalidomide was quickly recalled after this was discovered, it is still used today to treat things like leprosy and some cancers like multiple myeloma. However, it is very clear to doctors and patients that pregnant woman should not be prescribed this drug.
These cases are clear examples as to why it is very important to understand the structures and enantiomers of compounds before clearing them for public use and prescribing them. Chemists and scientists are well aware of the different biological effects of compounds with different structures and enantiomers, and they go through intensive research of these effects before getting them cleared by the FDA for therapeutic and public use. Luckily, in the case of enantiomers, scientists can sometimes find ways to separate the two R and S isomers in order to isolate the positive properties of a compound while avoiding negative side effects.
