It’s a hot, humid summer day; the sun is blazing down making waves of heat bounce off the pavement, the air is thick with moisture and there’s no sign of a breeze anytime soon. There are many ways to cool off in times like these: having a cold glass of lemonade, taking a refreshing dip in a pool, or even just staying inside planted next to the AC. But the best way to cool off is with a frozen dairy treat. In other words, ice cream.
I’m sure most people can recall countless hot days like this, and the giant wave of relief when the sound of the ice cream truck appeared in the distance, the anticipation growing as the sound grew closer. Although there are always numerous options of ice cream and popsicles, there is nothing more satisfying than a traditional ice cream sundae. And a crucial part of ice cream sundaes is the whipped cream – and the best whipped cream doesn’t come out of a can, it’s made fresh.
In honor of the summer season (or as I like to call it the “months of sundaes”) we took some images of whipped cream with an electron microscope to reveal the microscopic makeup of this delicious topping. Now that we have a closer look, let’s grab a spoon and dig in to the science behind whipped cream
The magic begins with the whipping process. When liquid cream is being whipped, millions and millions of tiny air bubbles start to form within the liquid. Although this process starts to thicken the cream and make it frothy, this early stage of whipping does not result in the final creamy product that we all know and love. If stopped too early, the bubbles would dissipate and the cream would go back to its initial liquid phase.
However, when the whipping process is continued, the cream begins to thicken more and more to a point where it cannot return to the liquid phase. This occurs because of the fats, called tr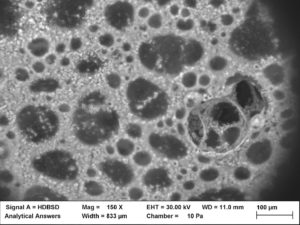 iglycerides, inside the cream. The triglyceride molecules in milk come with protective membranes, called phospholipids, similar to the shell on an egg. The whipping begins to break down these protective membranes on the triglyceride molecules. When the phospholipids are removed from the triglyceride membrane, they join together and then form protective barriers around the air bubbles. Then, when the air bubbles have their own protective membrane, they stick together and form larger and larger clumps, preventing them from dissipating and stopping the cream from going back to its liquid phase.
iglycerides, inside the cream. The triglyceride molecules in milk come with protective membranes, called phospholipids, similar to the shell on an egg. The whipping begins to break down these protective membranes on the triglyceride molecules. When the phospholipids are removed from the triglyceride membrane, they join together and then form protective barriers around the air bubbles. Then, when the air bubbles have their own protective membrane, they stick together and form larger and larger clumps, preventing them from dissipating and stopping the cream from going back to its liquid phase.
This means that whipped cream is technically an emulsion, or air suspended in a liquid and stabilized by fat. This is why whipping cream has to have a higher fat content in order for the whipped cream to form; if it doesn’t have enough fat, the air bubbles will dissipate and the cream will remain a liquid. This process is also similar to the formation of other whipped dairy products, like butter and ice cream.
So now that you know how whipped cream is made and what it looks like on a microscopic level, go ahead and enjoy a sundae topped with this whipped, fatty emulsion and think about all the little air bubbles inside of it as the spoon hits your tongue. And of course, don’t forget the cherry!
Sources:
http://www.thekitchn.com/food-science-how-whipped-cream-81751
https://www.uoguelph.ca/foodscience/book-page/whipped-cream-structure
https://foodretro.com/the-science-of-whipped-cream-and-butter/
